Vanda Pharmaceuticals: Growth, Challenges, and Innovation in 2025
Vanda Pharmaceuticals has cemented its reputation as a global leader in the biopharmaceutical sector. The company’s latest financial and operational updates shed light on growth areas and ongoing challenges while highlighting an innovative pharmaceutical pipeline.
Financial Highlights and Performance Overview
In the first quarter of 2025, Vanda Pharmaceuticals reported total net product sales of $50 million, representing a 5% increase compared to the same period last year. This growth was fueled primarily by the performance of Fanapt® and HETLIOZ®, offset by a decline in PONVORY® sales. Despite these gains, the company reported a net loss of $29.5 million, which was higher than the $4.1 million loss from Q1 2024. The increased loss mostly resulted from higher research and development expenses and a significant payment for a global license agreement.
For a detailed breakdown and further financial metrics, see the official First Quarter 2025 Financial Results released by Vanda Pharmaceuticals.
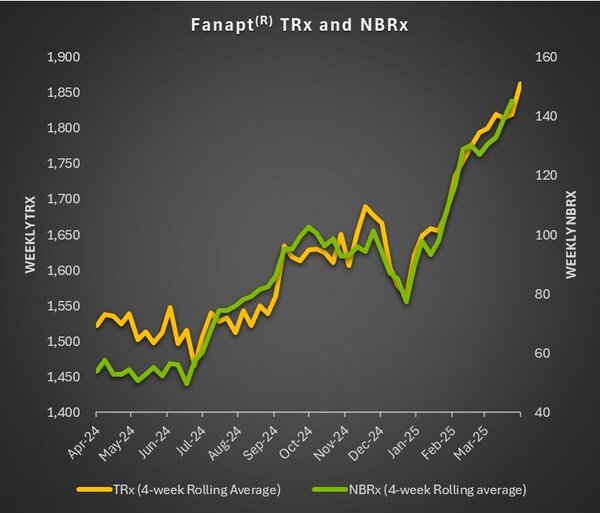
Fanapt: The Driving Force
Fanapt® (iloperidone) continued to shine as a core product. Q1 2025 saw a 14% increase in total prescriptions compared to Q1 2024, with new-to-brand prescriptions nearly tripling year over year. This boost followed Fanapt’s expanded approval for acute bipolar I disorder and a strategic increase in the company’s psychiatry sales force.
The strong market presence of Fanapt was recently highlighted in Vanda Pharmaceuticals’ earnings call analysis, which underscored growth in weekly prescriptions and the positive impact of new product launches.
Expanding the Pharmaceutical Pipeline
Vanda Pharmaceuticals is advancing a robust and diversified pipeline. Notable developments in 2025 include:
- Bysanti™ (milsaperidone): NDA accepted by the FDA for bipolar I disorder and schizophrenia, with exclusivity potentially extending into the 2040s.
- Tradipitant: NDA filed for motion sickness; clinical trials underway for additional indications.
- Imsidolimab: An IL-36R antagonist mAb under development for generalized pustular psoriasis, with a Biologics License Application planned this year.
- Vanda is also exploring new treatments in areas such as performance anxiety and Charcot-Marie-Tooth disease, further positioning the company as a pioneer in unmet medical needs.
Revenue Trends and Market Share Resilience
While Fanapt and HETLIOZ support the company’s top-line growth, PONVORY® sales saw an 18% year-over-year decline. Ongoing generic competition presents headwinds for HETLIOZ, but the product has maintained the largest portion of market share in its category. The company anticipates total revenues between $210 million and $250 million for full-year 2025 and expects solid cash reserves at year-end.
To learn more about Vanda Pharmaceuticals’ strategic direction, visit their latest earnings call recap, which examines both optimism and caution in today’s complex market.
Looking Forward: Innovation and Growth
Vanda Pharmaceuticals remains confident in its expansion plans. The company is focused on:
- Launching new therapies for central nervous system disorders and rare diseases
- Advancing late-stage clinical programs
- Reinforcing commercial operations across key franchises
Management expresses optimism for sustained revenue growth, increased new patient starts, and a vibrant future pipeline. As Vanda Pharmaceuticals navigates dynamic market landscapes, investors and healthcare professionals can expect continued innovation paired with a strong commitment to tackling high unmet needs.
Conclusion
Vanda Pharmaceuticals stands at the crossroads of opportunity and challenge in 2025. With powerful brands like Fanapt leading the charge and a well-filled drug pipeline, the company is poised for long-term growth. For the latest updates and strategic insights, stakeholders are encouraged to follow Vanda Pharmaceuticals’ official news releases and industry analyses.